Important Announcement: QVHD is closely monitoring the community spread of Coronavirus (COVID-19). Please direct all general questions to the state's hotline by dialing 2-1-1 or call 833-ASK-YNHH (833-275-9644). Anyone experiencing symptoms (fever, cough, shortness of breath) is strongly urged to contact their medical provider to seek treatment. For additional information, visit our information page at www.qvhd.org/coronavirus-preparedness or ct.gov/coronavirus.
Seasonal Influenza (Flu) Information
FLU SHOTS ARE THE BEST PROTECTION AVAILABLE FOR FIGHTING THE FLU
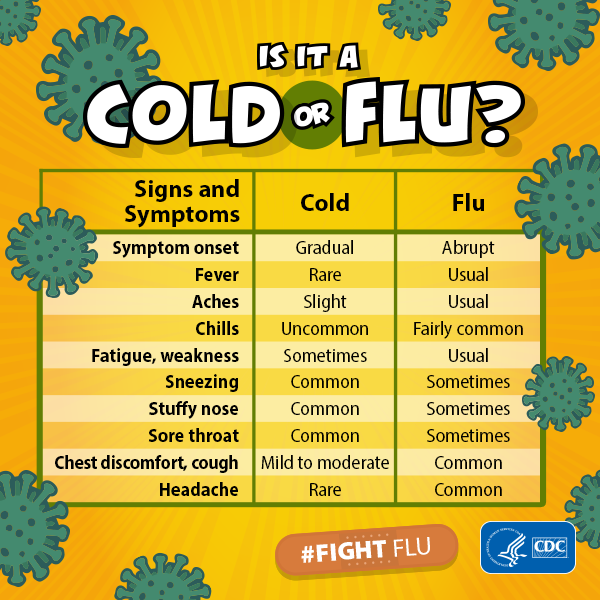
Influenza (flu) is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness. Serious outcomes of flu infection can result in hospitalization or death. Some people, such as older people, young children, and people with certain health conditions, are at high risk of serious flu complications. There are two main types of influenza (flu) virus: Types A and B. The influenza A and B viruses that routinely spread in people (human influenza viruses) are responsible for seasonal flu epidemics each year.
The best way to prevent flu is by getting vaccinated each year.
Who should get vaccinated this season?
Everyone 6 months of age and older should get an influenza (flu) vaccine every season with rare exception. CDC’s Advisory Committee on Immunization Practices has made this recommendation since the 2010-11 influenza season.
Vaccination to prevent flu is particularly important for people who are at high risk of developing serious flu complications. See People at High Risk of Developing Flu-Related Complications for a full list of age and health factors that confer increased risk.
More information is available at Who Should Get Vaccinated Against Influenza.
Where can you find a flu shot in your community?
THERE ARE MANY FORMS OF FLU IMMUNIZATIONS
Take a short quiz to determine which flu vaccine is best for you: https://vaccinefinder.org/recommendations/recform.php
Yes. There are different influenza vaccine manufacturers and multiple influenza vaccine products licensed and recommended for use in the United States.
CDC recommends use of any licensed, age-appropriate influenza vaccine during the 2019-2020 influenza season, including inactivated influenza vaccine [IIV], recombinant influenza vaccine [RIV], or live attenuated influenza vaccine (LAIV). No preference is expressed for any influenza vaccine over another. Both trivalent (three-component) and quadrivalent (four-component) influenza vaccines will be available.
Trivalent influenza vaccines include:
- A trivalent influenza shot made with adjuvant (Fluad), licensed for people 65 years and older.
- A high-dose influenza vaccine (Fluzone High-Dose), licensed for people 65 years and older.
Quadrivalent flu vaccines include:
- Standard-dose quadrivalent influenza shots that are manufactured using virus grown in eggs. These include Afluria Quadrivalent, Fluarix Quadrivalent, FluLaval Quadrivalent, and Fluzone Quadrivalent. Different influenza shots are licensed for different age groups. Some are licensed for children as young as 6 months of age. Most influenza shots are given in an arm muscle with a needle. One quadrivalent influenza shot (Afluria Quadrivalent) can be given either with a needle (for people aged 6 months and older) or with a jet injector (for people aged 18 through 64 years only).
- A quadrivalent cell-based influenza shot (Flucelvax Quadrivalent) containing virus grown in cell culture, which is licensed for people 4 years and older. This season, all four of the vaccine viruses used in Flucelvax have been grown in cells, making the vaccine totally egg-free.
- Recombinant quadrivalent influenza shot (Flublok Quadrivalent), an egg-free vaccine, approved for people 18 years and older.
There are many vaccine options to choose from, but the most important thing is for all people 6 months and older to get an influenza vaccine every year. If you have questions about which vaccine is best for you, talk to your doctor or other health care professional. More information on approved influenza vaccines for the 2019-2020 influenza season, and age indications for each vaccine are available in CDC’s Table: U.S. Influenza Vaccine Products for the 2019-20 Season
How do flu vaccines work?
Flu vaccines cause antibodies to develop in the body about two weeks after vaccination. These antibodies provide protection against infection with the viruses that are used to make the vaccine.
The seasonal flu vaccine protects against the influenza viruses that research indicates will be most common during the upcoming season. Most flu vaccines in the United States protect against four different flu viruses (“quadrivalent”); an influenza A (H1N1) virus, an influenza A (H3N2) virus, and two influenza B viruses. There are also some flu vaccines that protect against three different flu viruses (“trivalent”); an influenza A (H1N1) virus, an influenza A (H3N2) virus, and one influenza B virus. Two of the trivalent vaccines are designed specifically for people 65 and older to create a stronger immune response.
Are any of the available flu vaccines recommended over others?
No. For the 2019-2020 influenza season, CDC and its Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone 6 months and older with any licensed age-appropriate influenza vaccine including inactivated influenza vaccine (IIV), recombinant influenza vaccine (RIV4) or live attenuated influenza vaccine (LAIV4) with no preference expressed for any one vaccine over another.
Who Should Not Be Vaccinated?
Different influenza vaccines are approved for use in different age groups. In addition, some vaccines are not recommended for certain groups of people. Factors that can determine a person’s suitability for vaccination, or vaccination with a particular vaccine, include a person’s age, health (current and past) and any allergies to influenza vaccine or its components.
When should I get vaccinated?
You should get a flu vaccine before flu viruses begins spreading in your community, since it takes about two weeks after vaccination for antibodies to develop in the body and provide protection against flu. Make plans to get vaccinated early in fall, before flu season begins. CDC recommends that people get a flu vaccine by the end of October. Getting vaccinated later, however, can still be beneficial and vaccination should continue to be offered throughout the flu season, even into January or later.
Getting vaccinated early (for example, in July or August) is likely to be associated with reduced protection against flu infection later in the flu season, particularly among older adults.
Children who need two doses of vaccine to be protected should start the vaccination process sooner, because the two doses must be given at least four weeks apart.
While a flu shot is not a 100% guarantee that you will not get the flu this season, it is a safe, inexpensive and an effective method to reduce your chances of getting the flu. Please consider vaccination this season. Not only do you protect your own health, but you protect the health of those most vulnerable to complications from the flu.
IS THERE MEDICINE TO TREAT FLU?
Medicines, called antivirals, can treat flu illness. They can make people feel better and get better sooner. They may prevent serious flu complications, like pneumonia, that can lead to hospitalization and even death. These drugs are different from antibiotics, but like antibiotics, they also need to be prescribed by a doctor. They work best when started during the first 2 days of illness. It is very important that antiviral drugs be used early to treat flu in people who are very sick, such as people who are in the hospital or people who are at greater risk of having serious flu complications.
FOR MORE INFORMATION ON ANTIVIRALS FOR FLU, visit http://www.cdc.gov/flu/antivirals/whatyoushould.htm
THERE ARE ACTIONS YOU CAN TAKE TO PREVENT THE SPREAD OF FLU
Take 3: Behaviors to reduce the spread of flu and flu-like illness
Websites where you can find additional information about the flu: